The Premier Place for Cannabis Beauty and Business News
Tarrytown-based Regeneron is working to find a drug that has the potential to not only protect a person against the COVID-19 coronavirus, but to also treat a person who is already infected

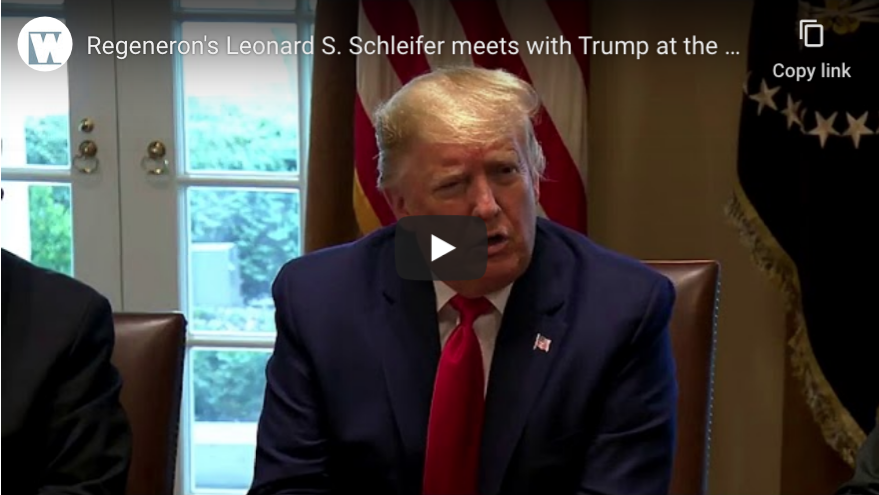
Tarrytown-based Regeneron is working to find a drug that has the potential to not only protect a person against the COVID-19 coronavirus, but also to treat a person who is already infected, the company’s founder, president and CEO told President Donald Trump during a March 2 meeting at the White House.
Dr. Leonard S. Schleifer, the founder, president and CEO of Regeneron, was one of several heads of research and pharmaceutical companies invited to a meeting of the president’s Coronavirus Task Force in the Cabinet Room. Vice President Mike Pence, Health and Human Services Secretary Alex Azar and Deborah Birx, the White House coronavirus response coordinator, were among those attending the meeting.
In early February, Regeneron reached an expanded agreement with the Department of Health and Human Services to develop new treatments for combating the coronavirus that has been spreading around the world at increasing speed. Regeneron is collaborating with HHS’ Biomedical Advanced Research and Development Authority (BARDA).
During the meeting, Trump pressed the heads of the companies to promise a quick timeline for putting a vaccine into production. Dr. Anthony Fauci, a member of the task force and director of the National Institute of Allergy and Infectious Diseases, had previously told the president that it would take a year to 18 months to invent a vaccine, do clinical trials and, if it actually worked, get Food and Drug Administration approval to bring it to market. Schleifer raised the possibility of a briefer timeline if a number of factors fell into place.
He explained that the company worked with BARDA and came up with a cure for Ebola.
“Dr. Fauci’s group was really instrumental in testing that under unbelievable conditions in the Congo,” Schleifer told the president.
Schleifer said Regeneron can use the same technology and is working to develop something effective against COVID-19.
Biotech company Regeneron

“We have 1,000 antibodies that are already sitting in dishes. We’re screening them. We’re selecting them. We anticipate, if all goes well, 200,000 doses per month can come out of our factory in New York, starting in August,” Schleifer told Trump.
The antibodies can block the proteins that allow the virus to latch onto human cells. Cells in the lungs are especially vulnerable to the COVID-19 strain of virus, which has similarities to Middle East Respiratory Syndrome (MERS) and Severe Acute Respiratory Syndrome (SARS) viruses.
Trump asked, “That means you’d be able to use the vaccine that early?”
Schleifer replied, “It depends on what we see, how we work closely with the FDA, which we will do. The FDA already reached out to us, but we’ve got to work closely.”
Azar asked why Regeneron could be on a faster timetable than some other companies working on vaccines or therapies and whose representatives were in the room.
“Our drug will be able to protect you,” Schleifer replied. “Whether or not you’re infected, it will protect you from getting infected. Or if you are infected, it would treat you. We have just taken processes that normally take years – literally, years – and we put them end to end and now do them in weeks to months, which nobody else in the industry can do.”
Trump interjected, “So this would be a companion of a vaccine and also it will – to put it a different way – make you better, quicker.”
Schleifer said, “If you get immunized with one of these vaccines, you’re going to make some antibodies to protect you. We’re going to already make those antibodies and give them to you so you don’t have to go through the whole process. So, it’ll protect you. And, as we showed with Ebola, if you give enough of them … it was lifesaving – truly lifesaving. And it beat out the antivirals (a different type of drug). It … was the way to go. It’s very predictable.
“I sense the cautiousness of Dr. Fauci and he’s right to be cautious, because vaccines have to be tested, because there’s precedence for vaccines to actually make diseases worse. You don’t want to rush and treat a million people and find out you’re making 900,000 of them worse.”
“That’s a good idea,” Trump replied.
“We know that this approach worked for Ebola. We know that it worked for MERS in animals. We have a greater degree of confidence that this would work sooner, I think,” Schleifer said, “We’re going to be surprised about what happens over the next couple of months.”
Trump asked if a flu vaccine would have any impact on the coronavirus. Both Schleifer and Fauci said “no.”
Source: https://westfaironline.com/121919/regeneron-uses-a-double-barreled-approach-on-covid-19/
Biotech company Regeneron
tags: ALEX AZAR, CORONAVIRUS TASK FORCE, COVID-19, DONALD TRUMP, LEONARD SCHLEIFER, MERS, REGENERON, SARS, VICE PRESIDENT PENCE, WHITE HOUSE
SECTIONS
The Premier Place for Cannabis Beauty and Business News
Events + GALLERY
The Premiere Place for Cannabis Beauty and Business News
sections & Articles
The Premiere Place for Cannabis Beauty and Business News
Recent Articles
SEARCH MORE TOPICS HERE
RECENT ARTICLES
The Premiere Place for Cannabis Beauty and Business News

How CBD-Infused Products Can Save Your Skin This Winter
by Katie Shapiro
Senior Contributor Vices
Whether you live in one of the world’s colder regions or are planning a ski vacation this season, the dry air that comes with winter — especially at higher altitudes — can take an extreme toll on your skin. As cannabis-infused products continue to take the beauty industry by storm, one lesser-known secret is that the plant can provide serious moisture, relief and protection against the damaging effects of the elements.
Despite new lines continually hitting the shelves at the likes of Sephora, there is still a lack of recent research and dermatologists have yet to publicly back brands. What we do know? The most common ingredient is hemp seed oil in the form of CBD extract, which is known to provide natural anti-inflammatory properties.
Dr. Jeanette Jacknin called hemp “the new cosmeceutical ingredient” during her “Hemp and Cannabinoids for Beauty and Skin Disorders” presentation at the American Academy of Dermatology’s annual meeting earlier this year. And now with the passing of the 2018 Farm Bill, which if signed by the president, will remove hemp from the Controlled Substances Act and make it a legal agricultural commodity again, the floodgates will fully open to the beauty and wellness industries.
While legislation progress is already underway, co-founder of L’Eela CBD Bodycare Amy Andrle explains, “Until cannabis as a whole is legalized on a federal level, a conflict of interest and hesitation to go on the record will remain for board certified doctors.”
Also a co-owner of the award-winning organic cultivator and retail dispensary L’Eagle Services in Denver and board member of the Cannabis Certification Council, Andrle is obviously a proponent for all-natural skincare products.
“Everyone’s composition is different, but in a lot of cases, skin can get worse when prescribed high concentrations of chemicals through traditional medicine,” says Andrle.
“One reason CBD-infused skincare is more effective is because hemp molecules are extremely small, making the penetration into the skin far easier than most creams,” according to Dr. Andrew Kerklaan, president and founder of Dr. Kerklaan Therapeutics.
Known for a rigorous triple-lab-testing process, Kerklaan drew on more than 20 years as a internationally-renowned chiropractor to combine both pain relief and healing properties into his product line, which he launched in 2017. “I’m hopeful that once we see regulatory things change, there will be more opportunity for more research and regulation in the sector. There are a lot of products getting produced that are over promising and under delivering, using irresponsibly sourced hemp,” says Kerklaan.
Until then, cannabis companies are only able to rely on customer feedback. Kerklaan says it’s compelling enough, citing the before and after photos he receives from around the world almost daily.

CoronaVirus: If Procter & Gamble Feels A Downturn In Performance, What Does That Mean For Cannabis Beauty Brands & Businesses?
by Cheryl Green
Most of America’s industries source from China in one form or another. Domestic cannabis brands use suppliers for different aspects of their businesses from all over the world, but China in particular is where most businesses turn when it comes to getting things at super low costs, which increases their profit margins.
If behemoth companies like P&G are feeling the negative impact that the coronavirus is having on their brands, imagine how it’s affecting the owners of cannabis business and beauty brands! They are already seeing a drastic change in the behavior of their customers and how they are and are not shopping.
Below, in an article from gcimagazine.com, Jon Moeller, vice chairman, COO and CFO of The Procter & Gamble Company weighs in on their challenges. Where the cannabis beauty and business sector is concerned, everyone needs to pay attention and prepare– starting now.
Coronavirus-Proctor & Gamble
February 21, 2020 Source: GCImagazine.com
Procter & Gamble has announced it’s facing coronavirus-related challenges.
Previously: PCHI Rescheduled | Further reading: Coronavirus Boosts Beauty Livestreaming
Jon Moeller, vice chairman, COO and CFO of The Procter & Gamble Company provided remarks at the Consumer Analyst Group of New York 2020 Conference, including the following comments:
“We face the demand and supply challenges associated with the coronavirus outbreak. China is our second largest market—sales and profit. Store traffic is down considerably, with many stores closed or operating with reduced hours. Some of the demand has shifted online but supply of delivery operators and labor is limited. There are also impacts outside of China: travel retail, a significant reduction in department store traffic in many Asian metro areas, and global supply. We access 387 suppliers in China that ship to us globally more than 9,000 different materials, impacting approximately 17,600 different finished product items. Each of these suppliers faces their own challenges in resuming operations. The operating challenges change with the hour, and of course the path of the virus is unknown, making it very difficult to provide precise estimates of impact.
Results for the January to March quarter in China and for the total company will be materially impacted on both the top and bottom line by these dynamics.
We continue to believe, based on what we know today, that our fiscal year top and bottom line guidance ranges—and I emphasize ranges—remain the right ones. We will continue to monitor the situation and obviously update you if and when a different reality becomes apparent.”
The company is furnishing this 8-K pursuant to Item 7.01, “Regulation FD Disclosure.”
Coronavirus-Proctor & Gamble